Baxter Infusor Manual
Posted By admin On 07.01.20BAXTER HEALTHCARE - SINGAPORE 6461500 INFUSOR PUMP (1 LABEL) INFUSION, PUMP Catalog Number 2L3100 Device Problem Failure to Deliver Event Date Event Type Malfunction Manufacturer Narrative (b)(4). The device is not available to baxter for evaluation at this time. Due diligence has been performed to acquire the pump. If the pump becomes available, a follow-up medwatch report will be submitted. Manufacturer Narrative (b)(4). The device has been requested for evaluation.

Should the device be received and an evaluation performed, a follow-up report will be filed. Event Description Materials manager at customers facility called product surveillance on 3/4/10 to report a problem with an infusor pump.
The materials manager reported the pump is set up to administer medication to patient with a terumo syringe. The set being used is baxter product (b)(4). The pump will alarm after 5cc of medication has been administered. The anesthesiologist then removes the syringe from the pump and manually administers 1cc of medication to patient. The syringe is then placed back in the pump, however the pump continues to alarm. The anesthesiologist then removes the syringe and manually administers the remaining amount of medication to complete patient's procedure. No patient injury or medical intervention has been reported.
No additional information is available. Brand Name 6461500 INFUSOR PUMP (1 LABEL) Type of Device INFUSION, PUMP Manufacturer (Section D) BAXTER HEALTHCARE - SINGAPORE 2 woodlands industrial park singapore 2573 SINGAPORE 2573 Manufacturer (Section G) BAXTER HEALTHCARE - SINGAPORE 2 woodlands industrial park singapore 2573 SINGAPORE 2573 Manufacturer Contact karen kirby 25212 w. Illinois route 120 round lake, IL 704541 MDR Report Key 1629813 Report Number 60-00297 Device Sequence Number 1 Product Code Report Source Manufacturer Source Type User facility Reporter Occupation Other Type of Report Initial,Followup Report Date 1 Device Was Involved in the Event 0 PatientS WERE Involved in the Event: Date FDA Received Is This An Adverse Event Report? No Is This A Product Problem Report?
Yes Device Operator Health Professional Device Catalogue Number 2L3100 Was Device Available For Evaluation? Yes Is The Reporter A Health Professional?
No Date Manufacturer Received Was Device Evaluated By Manufacturer? Device Not Returned To Manufacturer Date Device Manufactured Is The Device Single Use?
Office Assistant (General) & (Typing) Examination Study Guide. TABLE OF CONTENTS. Should study the content assessed in each section of the test. 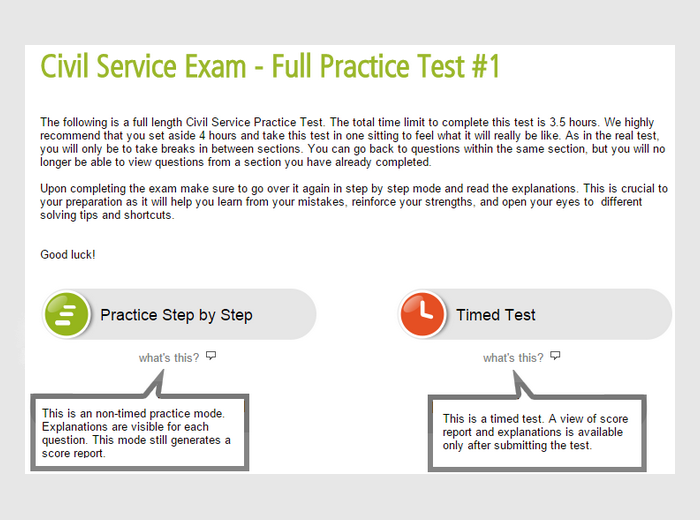 The Court Office Assistant Passbook® prepares you for your test by allowing. Or vocational licensing exam can find the test preparation guide they're looking. Passbooks® has been publishing test preparation study guides for more than 40 years, with 5,000 titles available in a wide range of fields. Passbooks® are. Court Assistant, Spring Supervising Court Office Assistant A thorough exam preparation includes practice tests, study guide, question & answer analysis. The Court Office Assistant Exam is a version of three other exams: the Court Assistant. New York City Bus Operator Exam Review Guide.
The Court Office Assistant Passbook® prepares you for your test by allowing. Or vocational licensing exam can find the test preparation guide they're looking. Passbooks® has been publishing test preparation study guides for more than 40 years, with 5,000 titles available in a wide range of fields. Passbooks® are. Court Assistant, Spring Supervising Court Office Assistant A thorough exam preparation includes practice tests, study guide, question & answer analysis. The Court Office Assistant Exam is a version of three other exams: the Court Assistant. New York City Bus Operator Exam Review Guide.
No Is this a Reprocessed and Reused Single-Use Device? No Type of Device Usage Reuse.
More Baxter InfusOR Pictures Baxter InfusOR Features The Baxter InfusOR pump is a syringe infusion device that will aid in the administration of many intravenous agents given during anesthetic procedures. In conjunction with the Smart Label system, it provides the convenient delivery of narcotics, muscle relaxants, vasoactives and other drugs routinely given during anesthesia. The Smart Label system is a set of drug-specific labels that attach to the front of the InfusOR infusion pump into a specific delivery system for the indicated drug with appropriate rate selections and delivery units. By simply changing specially coded smart label, the Bard InfusOR pump is automatically changed to a specific delivery system for a particular intravenous agent. The Baxter Bard InfusOR syringe pump is easy to setup and use. Each Smart Label is clearly marked with drug dilution instructions and the required syringe size. The pump accepts B-D or MonoJect 20 cc and 60 cc plastic disposable syringes which attach to tubing sets for connection to the patient’s primary intravenous line.
Drug delivery choices are easily selected and changed using front panel rotary switches. The selections are made directly in drug dosing units and, where appropriate, are related to patient body weight, thus elimination tedious calculations. The total volume of drug delivered is displayed for both patient management and record keeping needs. This lightweight battery-operated pump is provided with a versatile mounting bracket that securely fastens the pump and provides good pump accessibility during use. The Bard InfusOR Infusion Pump and Smart Label System combine the flexibility of a general infusion device with the convenience of a drug-specific infusion device to meet many intravenous delivery needs during anesthetic procedures.
Principles of Operation The InfusOR pump is a battery-operated device that holds and empties disposable syringes a in a controlled manner. A plastic holder accepts the syringe barrel. The syringe plunger is held and moved by a pusher assembly. The pusher engages a threaded leadscrew which is rotated by reliable, efficient motor that is controlled and monitored by a microcomputer system. Magnetically coded labels attach to the pump front panel and are read by the microcomputer. The coded label tells the microcomputer the delivery rate selections available when that specific label is used with the pump. Rotary input switches, set by the user, select the drug delivery rate.
Calculations are then performed by the microcomputer so the drug is delivered as indicated. A force sensing system responds to the overpressure in the fluid pathway and a position detector determines when the syringe needs refilling. The microcomputer controls the indicated lights and alarms and also displays the volume of drug delivered. The batteries are electronically monitored for a low power condition. Designed to aid in many intravenous agents given during anesthetic procedures. Provides for the convenient delivery of narcotics, muscle relaxants, vasoactives and other drugs routinely given during anesthesia.
Easy to use and set up. Drug delivery choices are easily selected and changed using front panel rotary switches. General Information About IV Therapy IV Therapy.
IV therapy is used to deliver by infusion, fluids, medication, or nutrients to patients. There are two main types of administration of infusion, volumetric and syringe/PCA. IV Therapy-Volumetric. These pumps are used when the accuracy of fluid volume to be infused is critical such as in the case of medications used for infusion of drugs for circulatory support. Volumetric pumps deliver a given volume of fluid per unit time measured in mL/hour. Volumetric pumps can be single or multi channel. Both types allow for the administration of more than one fluid at a time.
Single channel employs a piggyback method while multi-channel uses separate tubing set up for the administration of multiple medications or fluids. IV Therapy-Syringe. Syringe pumps are used to administer small volumes of fluids or medications.
Baxter Infusor User Manual
(1 mL to 60 mL syringe). Syringe pumps are an effective way to deliver medication with precision while reducing the chance of human error if the medication were to be administered by a member of the medical staff. (of course human error can occur when inputting data or choosing dose rate over time.). Like volumetric pumps, syringe pumps can be programmed to deliver the medication over a predetermined time. Baxter InfusOR Smart Labels The Smart Label is designed exclusively for use with the Baxter InfusO.R.
When attached to this Syringe Pump, the microprocessor will decode the magnetic sensor in the Smart Label to determine delivery rates. The InfusOR Pump will then deliver the drug in the syringe at the indicated infusion rates. Each label has a label number, for identification purposes, which is located on the upper right section of the Smart Label. The Infus OR Pump and the Smart Labels are intended for controlled rate delivery of small volume parenteral fluids. Each Smart Label is intended for use with the drug indicated on the label at the specified concentration for delivery. The dug is delivered from either a 20 cc or 60 cc syringe which is also indicated on the Smart label. Pump delivery rates are shown on each Smart label.
For labels that provide bolus delivery, have body weight settings between 30 and 100 kg. The Bolus delivery is determined by multiplying the drug concentration which is listed on the label. When the InfusOR pumps are first turned on with the smart label in place, the pumps liquid crystal display (LCD) will show the label number. Baxter Infus OR Parts Soma Technology offers parts for the Baxter Infus OR.

Are you looking for a locking pole clamp, syringe loading label, front case assembly, push block assembly, plunger screw, or more? Head on over to to find more. Soma Medical Parts not only carries infusion/syringe pump parts, but also carries parts for all the medical equipment we sell. If you need help identifying, purchasing, or recommendations for your medical equipment.